"Abstract
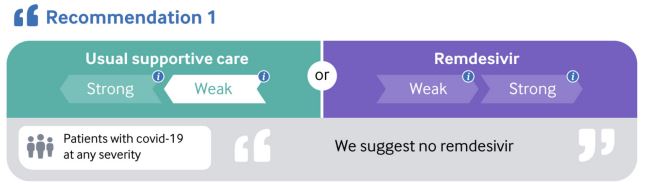
... New Recommendation: The latest version of this WHO living guidance focuses on remdesivir, following the 15 October 2020 preprint publication of results from the WHO SOLIDARITY trial. It contains a weak or conditional recommendation against the use of remdesivir in hospitalised patients with covid-19...
Understanding the New Recommendation: When moving from evidence to the conditional recommendation against the use of remdesivir in patients with covid-19, the panel emphasised the evidence suggesting no important effect on mortality, need for mechanical ventilation, time to clinical improvement, and other patient-important outcomes. Considering the low or very low certainty evidence for all outcomes, the panel interpreted the evidence as not proving that remdesivir is ineffective; rather there is no evidence based on currently available data that it does improve patient-important outcomes. The panel placed low value on small and uncertain benefits in the presence of the remaining possibility of important harms...
What triggered this version of the guideline?
This second version of the WHO living guideline addresses the use of remdesivir in patients with covid-19. It follows the preprint publication of the WHO SOLIDARITY trial on 15 October 2020, reporting results on treatment with remdesivir, hydroxychloroquine, and lopinavir-ritonavir in hospitalised patients with covid-19... The WHO SOLIDARITY trial adds 11 266 randomised patients (2570 to remdesivir, 954 to hydroxychloroquine, and 1411 to lopinavir-ritonavir, 6331 to usual care) and holds the potential to change practice...
The guidance
Remdesivir
The recommendation addressing remdesivir was informed by results from a systematic review and network meta-analysis that pooled data from four randomised trials with 7333 participants hospitalised
for covid-19."
Copyright © 2020, The Author(s). Published by BMJ Publishing Group Ltd.
This article is made freely available for use in accordance with BMJ's website terms and conditions for the duration of the covid-19 pandemic or until otherwise determined by BMJ. You may use, download and print the article for any lawful, non-commercial purpose (including text and data mining) provided that all copyright notices and trade marks are retained.
