Research letter
"[T]he possibility of batch-dependent variation appears worthy of investigation. We therefore examined rates of SAEs [serious adverse events] between different BNT162b2 vaccine batches administered in Denmark (population 5.8 million) from 27 December 2020 to 11 January 2022...
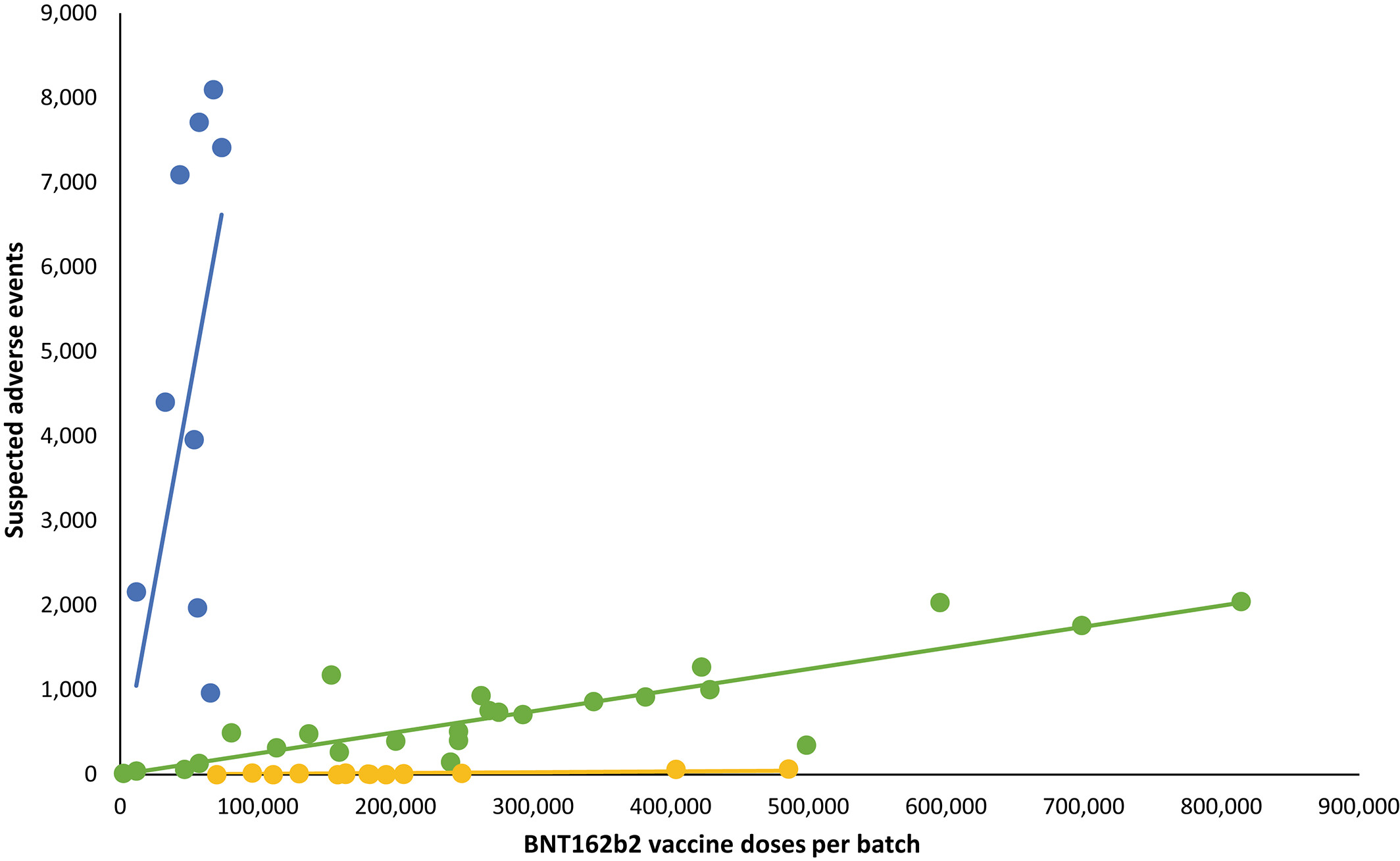
A total of 10,793,766 doses were administered to 4,026,575 persons with the use of 52 different BNT162b2 vaccine batches (2340–814,320 doses per batch) and 43,496 SAEs were registered in 13,635 persons, equaling 3.19 ± 0.03 (mean ± SEM) SAEs per person... Unexpectedly, rates of SAEs per 1000 doses varied considerably between vaccine batches with 2.32 (0.09–3.59) (median [interquartile range]) SAEs per 1000 doses, and significant heterogeneity (p < .0001) was observed in the relationship between numbers of SAEs per 1000 doses and numbers of doses in the individual batches. Three predominant trendlines were discerned, with noticeable lower SAE rates in larger vaccine batches and additional batch-dependent heterogeneity in the distribution of SAE seriousness between the batches representing the three trendlines (Figure 1). Compared to the rates of all SAEs, serious SAEs and SAE-related deaths per 1.000 doses were much less frequent and numbers of these SAEs per 1000 doses displayed considerably greater variability between batches, with lesser separation between the three trendlines (not shown).
The observed variation in SAE rates and seriousness between BTN162b2 vaccine batches in this nationwide study was contrary to the expected homogenous rate and distribution of SAEs between batches... [V]ariations (batch-to-batch, vial-to-vial and even dose-to-dose) in vaccines may occur as a result of variabilities and practice breaches in, for example vaccine manufacturing, storage, transportation, clinical handling and control aspects, and in 2021, three lots of the mRNA1273 vaccine totalling more than 1.6 million doses were recalled in Japan after 39 vials of the vaccine were found to contain foreign materials. Leaked and contested data have also suggested that some early commercial batches of the BNT162b2 vaccine contained lower than expected levels of intact mRNA."
© 2023 The Authors. European Journal of Clinical Investigation published by John Wiley & Sons Ltd on behalf of Stichting European Society for Clinical Investigation Journal Foundation.
This is an open access article under the terms of the Creative Commons Attribution-NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
