Research Letter
"This study uses Pfizer-BioNTech vaccine allocation dose data and SAE case data available through the Vaccine Adverse Event Reporting System (VAERS) [3] to identify associations between doses allocated and SAEs [suspected adverse events]...
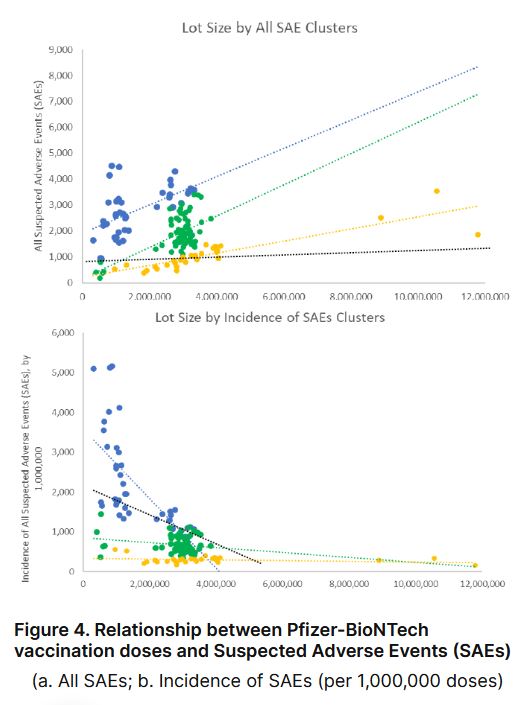
In conclusion, the batch-dependent trend in the U.S. is similar to trends in Denmark with some differences. Specific lot data is not directly comparable since different lots were allocated in the U.S.; however, a comparison using the same methodology provided similar results, namely that three clear clusters emerged. In general, U.S. data follows the same pattern however, in this study, all three clusters have slightly negative beta coefficients (decreasing SAEs with increasing lot size) where the Denmark study showed slightly positive (increasing SAEs with increasing lot size) (see Figure 4). The highest SAEs rates were identified in vaccine batches allocated in the first two months of the vaccination program, fitting squarely into the blue category, with the highest proportions being sent to government agencies, hospitals, universities, and health departments...
This study, like the Denmark study, concludes that there were clear variations in Pfizer-BioNTech’s vaccine lots and that the same pattern of SAEs was observed. The vaccination allocation data used in this study was only released through a forced legal action and does not provide data past April 2022."
Terms and Conditions
https://publichealthpolicyjournal.com/terms-and-conditions/
As an open-access journal, we ensure that all published articles are freely available to the global community, enhancing the visibility and impact of authors’ work. Options for copyright transfer by IPAK-EDU, IPAK PHP, and Science Public Health Policy & the Law are not available.
Manuscripts are published under the CC BY 4.0 DEED Attribution 4.0 International https://creativecommons.org/licenses/by/4.0/ by the author.
