"1. Introduction
... This narrative review focuses on various inherited thrombotic and coagulation disorders (inherited blood coagulation disorders and inherited thrombotic disorders in the light of COVID-19), and acquired thrombotic and coagulation disorders (blood coagulation and thrombotic disorders and bleeding complications following COVID-19 and COVID-19 vaccines), along with the possible pathogenesis hypotheses, therapeutic interventions, and imaging for diagnosing. We discuss possible pathophysiologic mechanisms interacting with the coagulation system during vaccination and review the currently available safety data regarding COVID-19 vaccines...
8. Thrombotic and Bleeding Events following COVID-19 Vaccination: Hypothesis for Pathogenesis and Therapeutic Interventions
... [A]t the beginning of 2021, the first cases of vaccine-induced thrombotic complications after vaccination with vector vaccines ChAdOx1 nCoV-19 (AstraZeneca) and Ad26.COV2.S (Johnson & Johnson/Janssen) were observed soon after all global vaccination programs were initiated. Subsequently, the relationship between thrombocytopenia and thrombosis after administration of BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) was also reported.
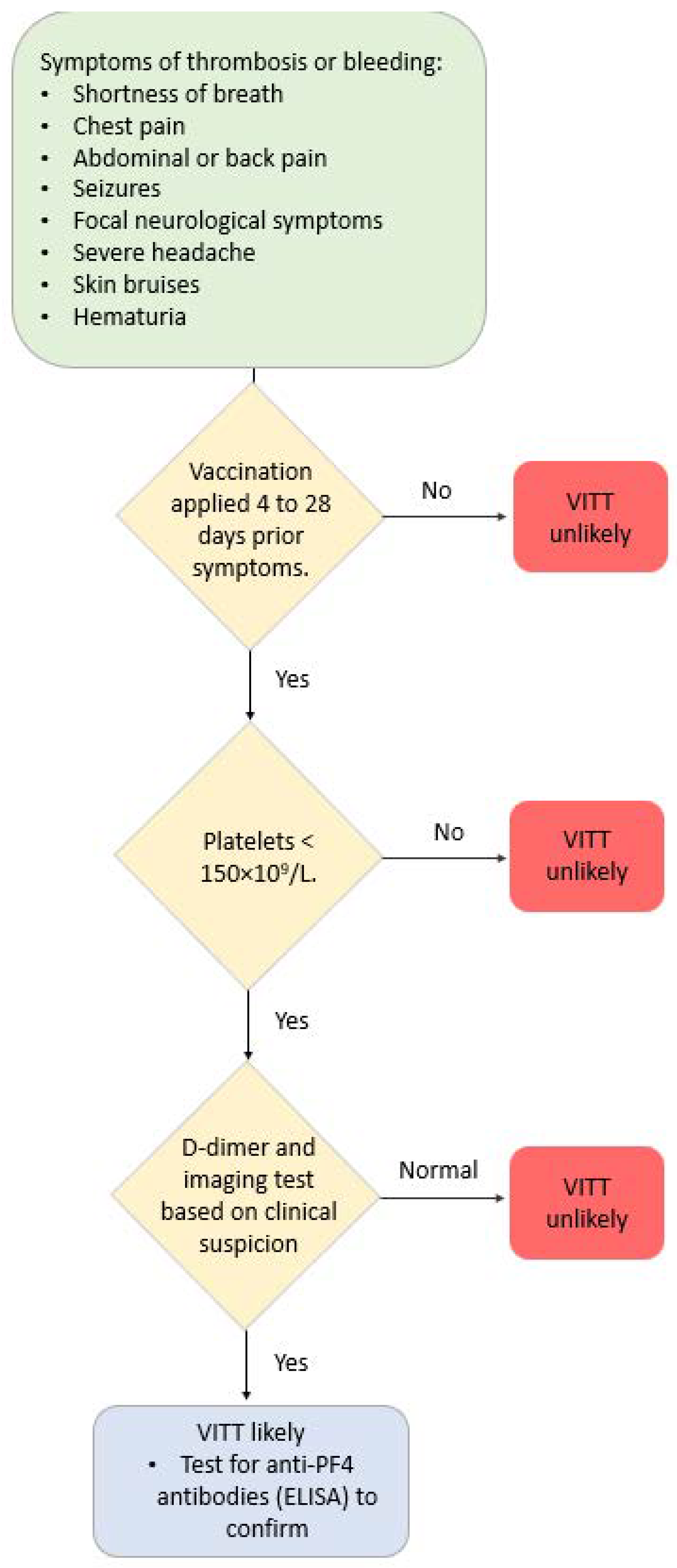
Different terms are used to refer to these conditions/complications after COVID-19 vaccination, which are predominantly localized in the venous vasculature system—vaccine-induced thrombotic thrombocytopenia (VITT), vaccine-induced pro-thrombotic immune thrombocytopenia (VIPIT), vaccine-induced immune thrombotic thrombocytopenia (VIITT) and thrombosis with thrombocytopenia syndrome (TTS). Antibodies that recognize PF4 (platelet-associated factor 4) cause these thrombotic events. The antibodies are IgG that activate platelets through specific receptors on the platelet surface. These events develop between 4 and 42 days after receiving a COVID-19 vaccine. Figure 2 illustrates a suggested diagnostic algorithm in patients with suspected VITT...
When adenoviral-based vaccines were invented and administered, reported cases of patients with thrombosis and thrombocytopenia were later found to have VITT. Venous thromboses in unexpected places, such as cerebral sinus vein thrombosis or splanchnic vein thrombosis, have been observed in COVID-19 vaccine recipients, occasionally with thrombocytopenia (thrombosis thrombocytopenia syndrome [TTS]). Vector-based COVID-19 vaccination increased TTS-related cerebral sinus vein thrombosis risks by 50-fold. These findings support a pathogenetic relationship between vector-based COVID-19 vaccinations and TTS. Vaccine antigenic complexes bind to platelet factor 4 (PF4) on platelet surfaces, causing proinflammatory responses, anti-PF4 antibody production, and pro-thrombotic cascades. Similarly, VITT is characterized by the production of platelet-activating antibodies targeting PF4. This clinical manifestation bears a resemblance to autoimmune heparin-induced thrombocytopenia.
Non-vector-based immunization was also related to cerebral thrombosis but without TTS."
Figure 2. Diagnostic algorithm in patients with suspected VITT.
© 2023 by the authors. Licensee MDPI, Basel, Switzerland.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
