Gabriele Segalla, PhD, is head of the MULTICHEM research and development laboratory and has created a portfolio of products from cosmetics to household formulations. He is the holder of patents in the field of organic chemistry and plant derivatives.
¨Abstract
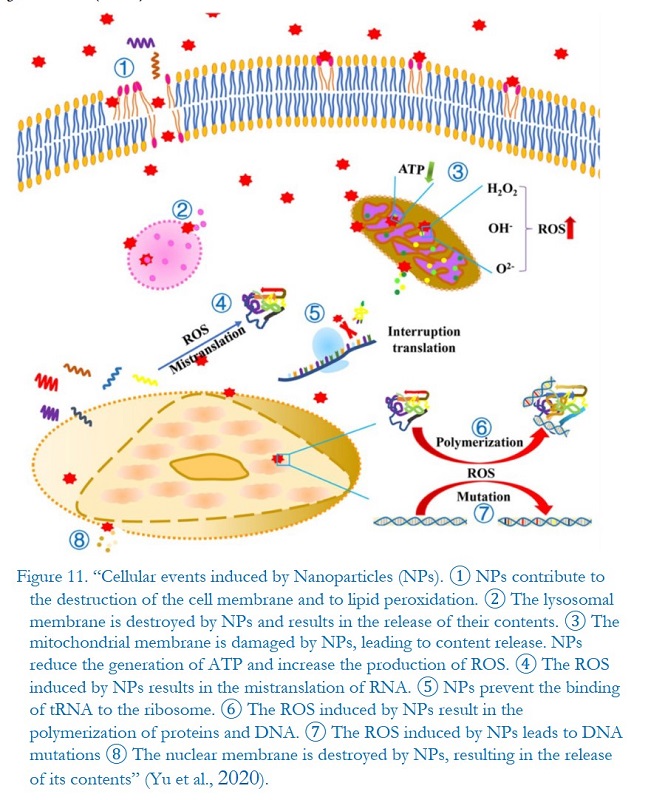
The medicinal preparation called Comirnaty by Pfizer-BioNTech is an aqueous dispersion of lipid nanomaterials, intended to constitute, after thawing and dilution, the finished product for intramuscular injection. In the present study, we examine some evident chemical-physical criticalities of the preparation, regarding the manifest instability of its qualitative-quantitative composition, as well as its consequent toxicological potential, in this case related to the possible formation of ROS (reactive oxygen species), after intramuscular inoculation, in different biological sites, such as, potentially, kidneys, liver, heart, brain, etc., causing dysfunctions and alterations thereof...
Conclusions
The Comirnaty COVID-19 mRNA BNT162b2 vaccine... presents numerous critical issues and drawbacks, examined in detail in this study and summarized as follows:
- The two functional excipients responsible for the formation of lipid nanoparticles, ALC-0315 and ALC-0159, are not registered in any Pharmacopoeia...
- Therefore, their toxicological profile is not known...
- Not all the chemical-physical analysis procedures and toxicological tests required for nanoforms of these substances have been carried out...
- Carcinogenicity, genotoxicity and mutagenicity studies of the preparation have not been carried out with the consent of the certifying body...
- The Safety Data Sheets of the Comirnaty preparation do not report information on the characteristics of the nanoforms present in the composition of the preparation itself...
- The actual values of the Polydispersity index and the Zeta potential of the nanoparticles present in the preparation are unknown. This leads to absolute uncertainty in the determination of the chemical-physical stability of nanoparticles and their aggregates...
- The presence of electrolytes in the original preparation... leads even more to the presumption that the product called Comirnaty PBS/Sucrose may give rise to the formation of aggregates and agglomerates before, during or after the inoculation procedure, and that it may therefore be both ineffective... and dangerous, as it would be deposited in tissues or organs not foreseen in its primary biological fate.
In conclusion, it is considered urgent and indispensable that an accurate and long-term study be carried out in the appropriate institutional, clinical or medico-legal seats, especially in relation to any causal or con-causal links between what is presented here and the wide pathological heterogeneity of serious or lethal adverse events that have occurred, or are occurring, after vaccinations, in order to adopt and expedite all appropriate corrective and preventive actions to protect public health... in accordance with the precautionary principle, and in the light of Article 10 of the Nuremberg Code."
Copyright (c) 2023 Gabriele Segalla
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
