“Abstract: This population-based retrospective cohort study assesses rates of adverse events (AE) after COVID-19 vaccines experienced by women of reproductive age, focusing on pregnancy and menstruation, using data collected by the Vaccine Adverse Events Reporting System (VAERS) database from Jan 1, 1998, to Jun 30, 2022.
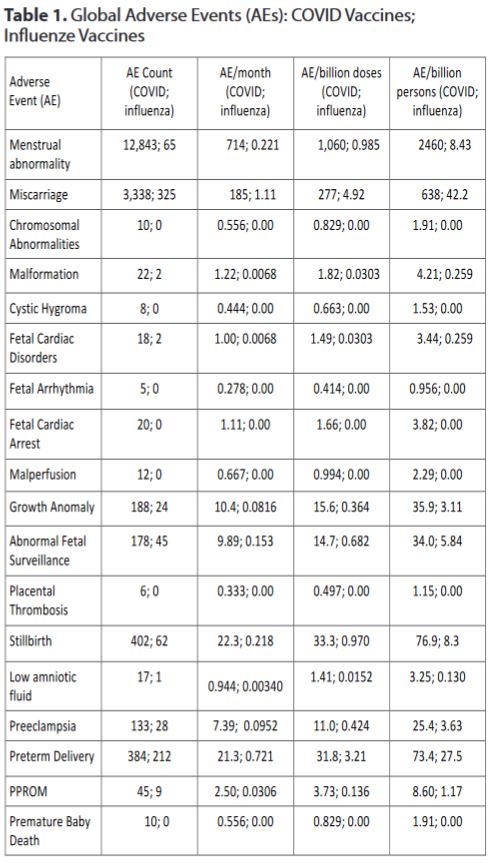
The proportional reporting ratio comparing AEs reported after COVID-19 vaccines with those reported after influenza vaccines is significantly increased (≥ 2.0) for COVID-19 vaccine for menstrual abnormality, miscarriage, fetal chromosomal abnormalities, fetal malformation, fetal cystic hygroma, fetal cardiac disorders, fetal cardiac arrest, fetal arrhythmias, fetal vascular malperfusion, fetal growth abnormalities, fetal abnormal surveillance, placental thrombosis, fetal death/stillbirth, low amniotic fluid, preeclampsia, premature delivery, preterm premature rupture of membrane, and premature baby death.
When normalized by time-available, doses-given, or number of persons vaccinated, all COVID-19 vaccine AEs far exceed the safety signal on all recognized thresholds.
These results necessitate a worldwide moratorium on the use of COVID-19 vaccines in pregnancy.”
Instructions for Authors: Journal of American Physicians and Surgeons
https://www.jpands.org/vol28no1/instructions.pdf
Copyright and Reprints: AAPS holds the copyright on all material published in the Journal. Permission is generally granted to reprint material from the Journal of American Physicians and Surgeons, providing that the material is not altered, and the Journal is properly cited, with date, authorship, copyright notice, and postal address and URL of AAPS.
