“Abstract
Background: The purpose of this study was to evaluate whether a bivalent COVID-19 vaccine protects against COVID-19.
Methods: The study included employees of Cleveland Clinic in employment when the bivalent COVID-19 vaccine first became available. Cumulative incidence of COVID-19 over the following 26 weeks was examined.…
Results: Of 51 982 eligible study participants, 965 (1.9%) were excluded because of missing age or sex. Of the remaining 51 017 employees included, 3294 (6.5%) were censored during the study because of termination of employment. By the end of the study, 13 134 (26%) had received the bivalent vaccine, which was the Pfizer vaccine in 11 397 (87%) and the Moderna vaccine in the remaining 1700. In all, 4424 employees (8.7%) acquired COVID-19 during the 26 weeks of the study.
Risk of COVID-19 Based on Prior Infection and Vaccination History
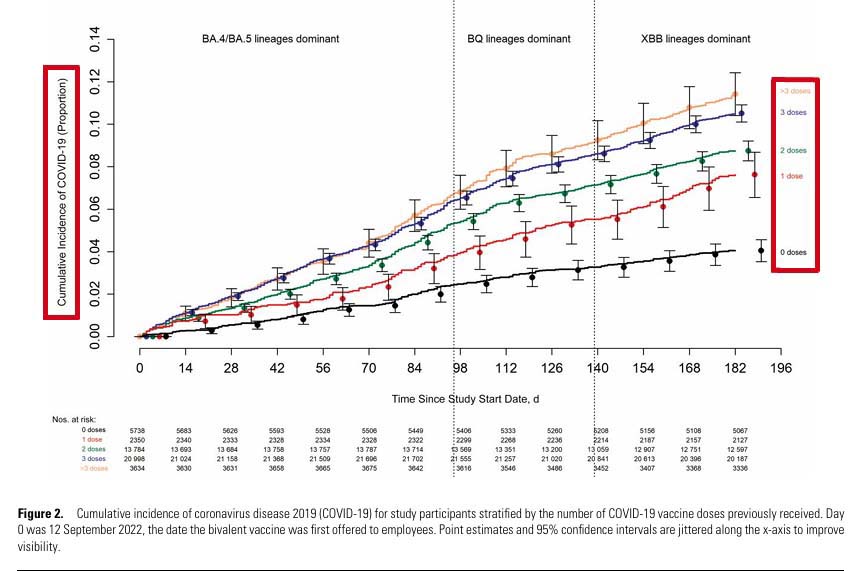
The risk of COVID-19 varied by the phase of the epidemic in which the study participant's last prior COVID-19 episode occurred. In decreasing order of risk were those never previously infected, those last infected during the pre-Omicron phase, and those last infected during the Omicron phase (Figure 1). The risk of COVID-19 also varied by the number of COVID-19 vaccine doses previously received. The higher the number of vaccines previously received, the higher the risk of contracting COVID-19 (Figure 2).
Discussion
In conclusion, this study found an overall modest protective effect of the bivalent vaccine against COVID-19 while the circulating strains were represented in the vaccine and lower protection when the circulating strains were no longer represented. A significant protective effect was not found when the XBB lineages were dominant. The unexpected finding of increasing risk with increasing number of prior COVID-19 vaccine doses needs further study."
© Crown copyright 2023. This Open Access article contains public sector information licensed under the Open Government Licence v3.0 (https://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/).
You are free to:
- copy, publish, distribute and transmit the Information;
- adapt the Information;
- exploit the Information commercially and non-commercially for example, by combining it with other Information, or by including it in your own product or application.
