"Background
... AstraZeneca's vaccine ChAdOx1 nCoV-19 was not known to cause vaccine-induced immune thrombotic thrombocytopenia (VITT) at the time of this case.
Case Presentation
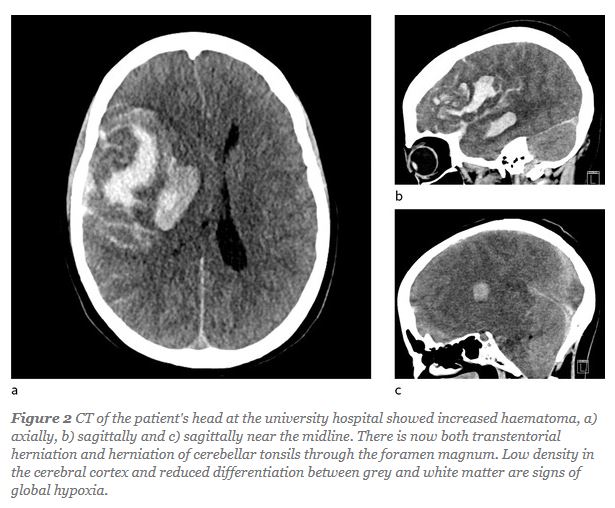
The patient was a previously healthy woman in her thirties with headaches that developed one week after vaccination with ChAdOx1 nCoV-19. Three days later, her condition deteriorated rapidly, and she presented to the emergency department with slurred speech, uncoordinated movements and reduced consciousness. Symptoms progressed to left-sided hemiparesis and her level of consciousness deteriorated. Computed tomography (CT) of the head showed a large right-sided haemorrhage and incipient herniation. She was found to have severe thrombocytopenia... In spite of efforts to reduce intracranial pressure, the patient died the following day...
Discussion
... A few days after this incident, Oslo University Hospital, Rikshospitalet reported multiple cases of severe blood clots and bleeding in patients who had received an identical vaccine. These patients also had low platelet counts, and in these cases a link was found between the events and the vaccine. Since then, the condition has been referred to as vaccine-induced immune thrombotic thrombocytopenia (VITT), which is characterised by low platelet counts, thrombus formation and antibodies to PF4. In light of this knowledge, new investigations were carried out, and our patient was also found to have a tendency towards thrombus formation with small thrombi in the transverse sinus, frontal lobe and pulmonary artery. Antibodies to PF4 were also detected. Overall, there is therefore a strong indication that this was a case of VITT. Retrospectively, it has to be asked whether the bleeding seen on the CT images represented a venous haemorrhagic infarction similar to that seen in several patients at Rikshospitalet, and whether the bleeding component may have been predominant as a result of VITT. A venous infarction might explain the patient's headache...
Several countries are now reporting similar incidents after vaccination... Following these incidents, the Norwegian Institute of Public Health issued a warning about potentially serious adverse effects of the vaccine. The AstraZeneca vaccine is now on hold in Norway. The government has appointed an expert panel to carry out a comprehensive risk assessment before any potential re-introduction of the vaccine. The Norwegian Institute of Public Health has reported that as the number of deaths from COVID-19 is now low in Norway, it seems that vaccination with the AstraZeneca vaccine entails a higher risk of death, particularly for younger people, than the risk of dying of the disease."
Copyright: The author(s)
Open access CC-BY-ND
