¨Abstract
In Spring 2021, MyCycleStorySM launched a secure online survey to which 92.3% of 6049 respondents self-reported menstrual irregularities occurring after the rollout of the COVID-19 injectables. Each respondent served as her own control because prior to the rollout of COVID-19 vaccination, the vast majority had regular menstrual cycles. A subgroup of 3390 respondents were only indirectly exposed to COVID-19 vaccines or the SARS-CoV-2 virus. This subgroup reported 1) being unvaccinated for COVID-19; 2) having had no COVID-19 symptoms; and 3) no positive test for COVID-19, yet a substantial majority of these women, who were only indirectly exposed to COVID-19 injectables or COVID-19 infections, still had many of the same menstrual abnormalities as the 2659 women who were directly exposed to a COVID-19 injection (798), or had COVID-19 symptoms (1347), or tested positive for COVID-19 (514)... Indirect exposure to COVID-19 vaccinated persons was significantly associated with the likelihood of the onset of menstrual irregularities. This study provides additional data to complement a growing body of evidence raising concerns regarding the safety of mRNA vaccines...
Introduction
... These mRNA countermeasure technologies are categorized as gene therapies by the Food and Drug Administration, the manufacturer descriptions, and in the scientific literature. The FDA defines gene therapy products as 'all products that mediate their effects by transcription and/or translation of transferred genetic material and/or by integrating into the host genome and that are administered as nucleic acids, viruses, or genetically engineered microorganisms'. This distinction from ´classic vaccines´ is important because regulatory agencies in the US and Europe strongly recommend pre- and post- clinical pharmacokinetic studies of gene therapy products to assess their safety prior to approval. The FDA provides detailed guidance for the ´design and analyses of shedding studies for virus or bacteria-based gene therapy´...
Shedding Phenomena
In addition, as described by Banoun (Banoun 2023), pharmacokinetic studies should include the study of the absorption, distribution, and biotransformation of the vaccine ingredients, the excretion (i.e. shedding) of mRNA containing lipid nanoparticles (LNPs), modified spike-encoding free mRNA, the resultant spike protein product manufactured by the human body, the residual DNA from the manufacturing process, and other chemical components. Unfortunately, there is no evidence that such shedding studies were implemented...
In response to increasing numbers of menstrual irregularity reports, several retrospective clinical studies have been performed, resulting in a general acceptance of a link between COVID-19 vaccination and menstrual irregularities...
Relevance to Menstrual Irregularities
None of the clinical trials for the COVID-19 mRNA vaccines reported on significant health effects specific to women, including possible reproductive harm and/or menstrual disorders...
The MyCycleStory℠(MCS) research collaborative is an assemblage of experienced research scientists, data management specialists, and obstetricians/gynaecologists from several regions of the United States. The MCS research collaborative created an online survey in 2021 to gather data to better understand this phenomenon with a long-term goal of uncovering both the cause and treatment solutions...
Results
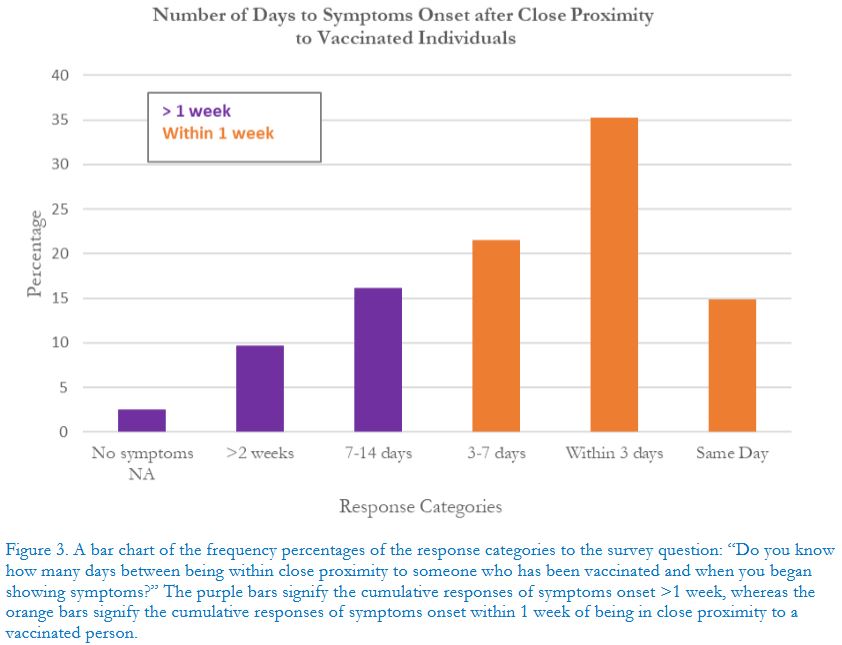
... One of our most striking findings was that 92.3% of the indirect exposure sample said that their abnormal or irregular first-time symptoms started after January 2021, including the 46-54 year-olds at 94%...
Discussion
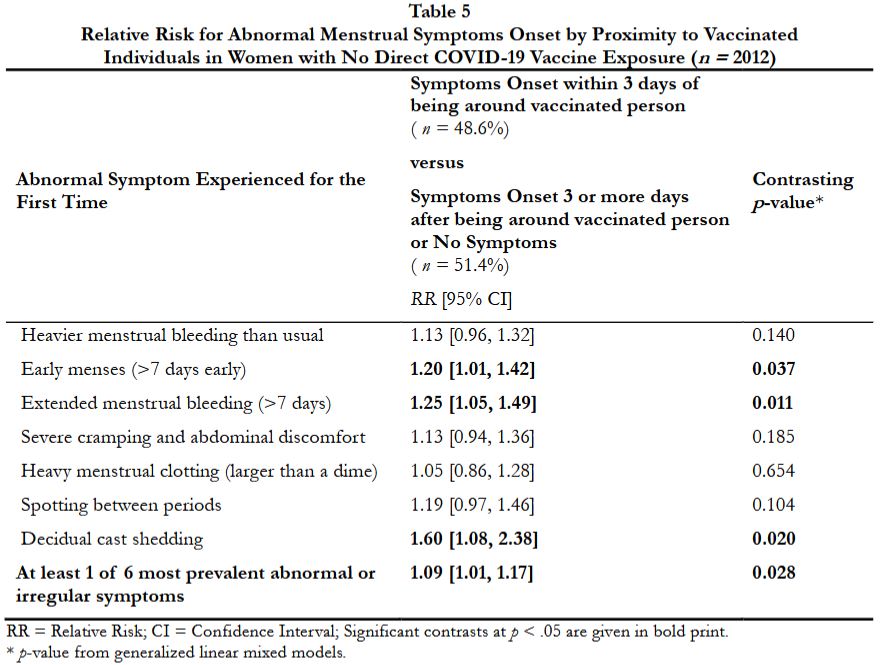
To our knowledge, this is the first peer-reviewed study that shows an increased risk for menstrual irregularities inindividuals who had not received COVID-19 vaccinations and, to their knowledge, were not exposed to the COVID-19 vaccine ingredients but were in close proximity to individuals who had received one or more injections of the COVID-19 vaccines...
Conclusion
Our findings suggest possible indirect transmission of ingredients or products of the COVID-19 vaccines, presumably through shedding, from people who received one or more of the COVID-19 injections."
The material presented is freely offered to all users who may take an interest in examining it, but how they may choose to apply any part of it, is the sole responsibility of the viewer/user. If material is quoted or reprinted, users are asked to give credit to the source/author, and to conform to the non-commercial, no derivatives, requirements of the Creative Commons License 4.0 NC ND or to any other license that takes precedence over it.
