Result of a Freedom of Information Request.
“Pharmacokinetics: Pharmacokinetic studies are generally not required for a vaccine per relevant guidelines; however, they are recommended for novel excipients or adjuvants used in the vaccine formulation, and in some cases for the antigen. The LNP in BNT162b2 contains two novel excipients, pharmacokinetics of which were studied in animal species and in vitro. In addition, tissue distribution of luciferase expressed by luciferase-encoding mRNA as a surrogate of the vaccine mRNA in the LNP formulation was also studied…
4.2.2. Study 185350
The distribution of lipid nanoparticles (containing ALC-0315 and ALC-0159) encapsulating mRNA encoding luciferase, was investigated by monitoring of a radiolabelled (3H-) lipid-marker after IM administration to Wistar rats.
Study details
Lipid nanoparticle formulation [LNP size, lipid composition (relative to mRNA concentration), and encapsulation efficiency similar to LNP in BNT162b2 vaccine] along with trace amounts of radiolabelled lipid marker cholesteryl-1,2-3H(N)]-cholesteryl hexadecyl ether (Figure 4-6), was administered intramuscularly to 42 Wistar Han rats (21/sex; 8-11 week age) at a target dose of 50 µg mRNA/animal (1.29 mg total lipid/animal)…
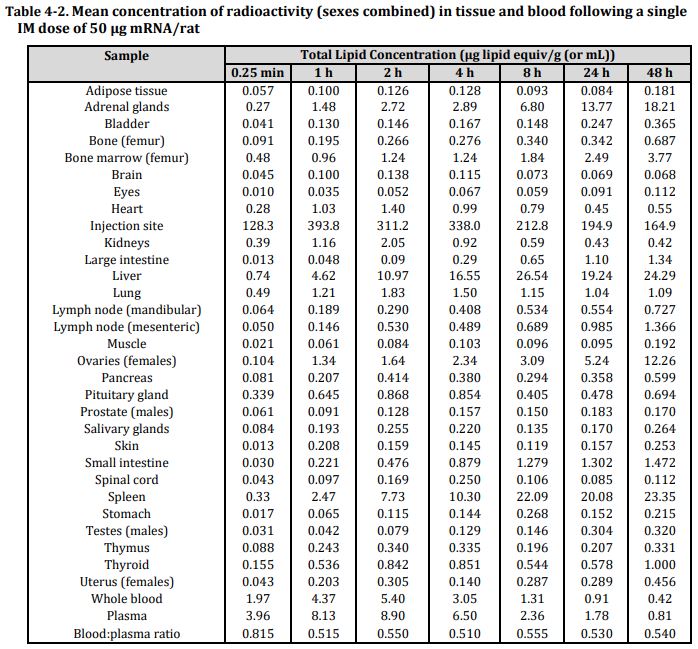
Tissue distribution
- The concentration of radioactive lipid marker reached the peak level in plasma (8.9 µg lipid eqv/mL) between 1 – 4 h post-dose and distribution mainly into liver, adrenal glands, spleen and ovaries over 48 h (Table 4-2). The concentration of radioactivity remained highest in injection site at all time-points…
- Mean total radioactivity was greatest at the injection site followed by the liver with much lower total recovery in spleen, adrenal glands and ovaries (Table 4-2)…
Conclusions
- Slow but significant distribution of lipid nanoparticles from the site of injection with major uptake into liver.
- Minor distribution in spleen, adrenal glands and ovaries over 48 h.
- Mean blood:plasma ratios of 0.5-0.6 indicating nanoparticles mainly present in plasma fraction of blood with peak concentrations in plasma at approx. 2 h post-dose.”
© Commonwealth of Australia
Copyright
https://www.tga.gov.au/copyright
Permission is given for fair dealing with this material including for the purposes of private study and research and may be reproduced as permitted under the Copyright Act 1968. (A copy of the Act is available at ComLaw - external site, the legal information retrieval system owned by the Australian Attorney General’s Department). All other rights are reserved.
Any permitted reproduction made must acknowledge the Commonwealth as the source of any selected passage, extract, diagram or other information or material reproduced. Any reproduction made of the information or material must include a copy of the original copyright and disclaimer notices as set out here.
