"Methods
Study cohort: The study was designed as an industry-independent, multicentre prospective cohort study to compare the safety and side effects of BNT162b2 vaccine in children aged 5-11 years with and without comorbidities ...
Results
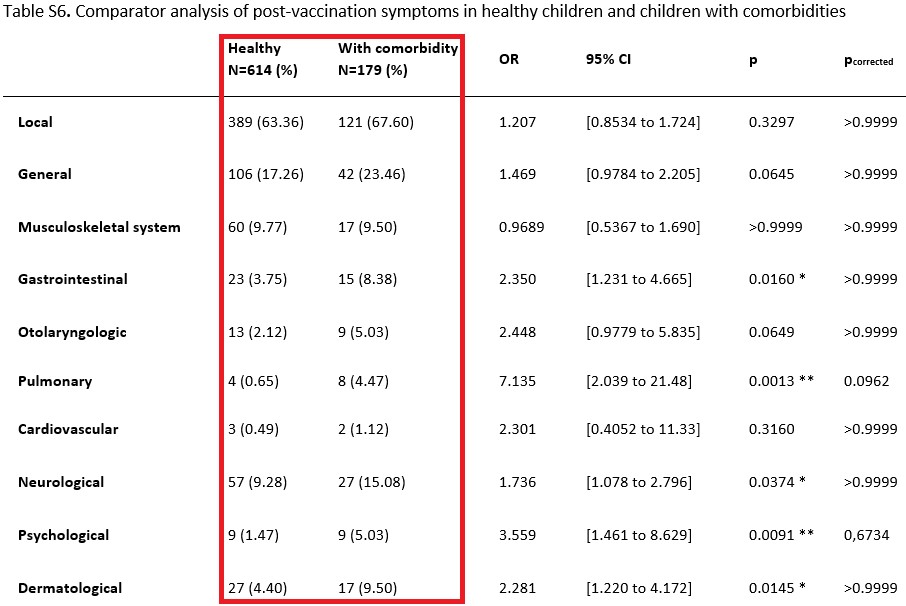
Frequency of post-vaccination symptoms: ... Comorbidities included pulmonary, malignant, rheumatologic, cardiological, genetic and gastrointestinal diseases, as well as primary immunodeficiencies and other diseases ...
Discussion ... Children with comorbidities report on more side effects after vaccination with BNT162b2 than healthy children. These side effects include especially gastrointestinal, otolaryngologic, pulmonary, neurological, psychological and dermatological symptoms. For example, children with comorbidities were 4.3 times more likely to experience nausea and vomiting after receiving a BNT162b2 vaccination than healthy children. Also, e.g. fever (defined as measured body temperature ≥ 38.5 °C) was more often reported in children with comorbidities than in healthy children. An underlying pathomechanism behind several side effects has been hypothesized to be a disturbance in the ACE-2/RAAS system by the spike protein produced after vaccination with BNT162b2, including as a result endothelial dysfunction. Also, effects of pro-inflammatory cytokines e.g. leading to altered protein synthesis have been described. The reasons for the striking differences in safety profiles of COVID-19 vaccine between healthy children and children with comorbidities remain unclear ...
Conclusion ... Especially since specific vaccination recommendations for children with comorbidities exist, safety and efficiency of BNT162b2 vaccination in children with comorbidities should further be evaluated. In general, it should be made mandatory for the respective pharmaceutical enterprises to perform studies in children with comorbidities, in particular if it becomes evident that recommendations for vaccination will focus on risk groups."
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
