In accordance with Transparency Regulation 1049/01, 130 doctors submitted a request for all pharmacovigilance safety data for all COVID-19 vaccines. In accordance with Transparency Regulation 1049/01, the European Medicines Agency (EMA) released this document.
Reporting period: December 19, 2021 through June 18, 2022
"6.3 Cumulative and Interval summary Tabulations from Post-Marketing Data Sources
Appendix 2.2 provides a cumulative and interval summary tabulation of adverse drug reactions by PT [preferred term] from post-marketing sources...
6.3.1.1. General Overview of the Safety Database - All Cases ...
General Overview - All Cases
A total of 508,351 case reports ... containing 1,597,673 events fulfilled criteria for inclusion in this PSUR [periodic safety update report] reporting period..."
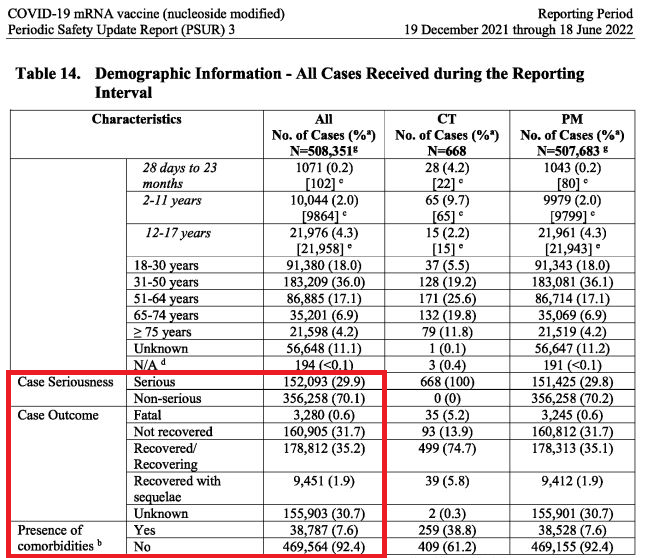
"6.3.1.1.2. General Overview of the Safety Database - Post-Authorization Data
During the reporting period, in the PM [post-marketing] dataset the number of female subjects was 2.2 times the number of male subjects (63.8% vs 29.4%)...
Adverse Event Data
A total of 1,596,793 AEs (of which 439,443 were serious and 1,158,240 non-serious) were reported in 507,683 PM cases.
The MedDRA SOCs containing the greatest number of events (≥2%) were General disorders and administration site conditions (459,731), Nervous system disorders (204,185), Musculoskeletal and connective tissue disorders (148,849), Injury, poisoning and procedural complications (130,333), Infections and infestations (82,131), Gastrointestinal disorders (81,816), Reproductive system and breast disorders (77,917), Skin and subcutaneous tissue disorders (62,405), Respiratory thoracic and mediastinal disorders (56,663), Cardiac disorders (54,208), Surgical and medical procedures (52,531), and Blood and lymphatic system disorders (38,366)."
PSUR4doctors: Doctors force EMA to release thousands of pages of Covid vaccine safety data, by Dr. Peter F. Mayer
https://tkp.at/2023/10/17/psur4doctors-aerzte-zwingen-ema-zur-herausgabe-tausender-seiten-covid-impfstoff-sicherheitsdaten/
Why does the EMA withhold disclosed documents as if they were a state secret? by Dr. Silvia Behrendt
https://tkp.at/2024/10/23/warum-haelt-die-ema-offengelegte-dokumente-wie-ein-staatsgeheimnis-zurueck/
Released by the EMA in accordance with Transparency Regulation 1049/01.
