“I. Introduction: Plaintiffs move under Rule 65, Fed.R.Civ.P., for a preliminary injunction against Defendants enjoining them from continuing to authorize the emergency use of the so-called ‘Pfizer-BioNTech COVID-19 Vaccine,’ ‘Moderna COVID-19 Vaccine’ and the ‘Johnson & Johnson (Janssen) COVID-19 Vaccine’ (collectively, the ‘Vaccines’) pursuant to their respective EUAs, and from granting full Food and Drug Administration (“FDA”) approval of the Vaccines.’…
A. The Unlawful Vaccine Emergency Use Authorizations
(1) 21 U.S.C. § 360bbb–3(b)(1)(C): There is No Emergency...
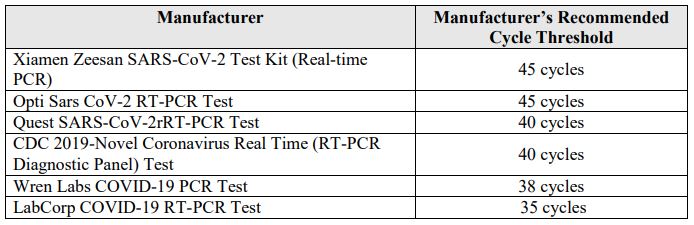
Virtually all scientists, including Dr. Fauci, agree that any PCR test run at a CT value of 35-cycles or greater is useless...
A study funded by the French government showed that even at 35-cycles, the false positivity rate is as high as 97%. Despite this, a majority of the PCR tests for COVID-19 deployed under EUAs in the United States are run at 35-45 cycles in accordance with manufacturer instructions. Under the EUAs issued by the FDA, there is no flexibility to depart from the manufacturer's instructions and change the way in which the test is administered or interpreted. The chart below shows that all major PCR tests in use in the United States are run at cycles of up to 35 or higher...
(3) § 360bbb–3(c)(2)(A): The Vaccines Do Not Diagnose, Treat or Prevent SARS-CoV-2 or COVID-19…
In studying the effectiveness of a medical intervention in randomized controlled trials (often called the gold standard of study design), the most useful way to present results is in terms of Absolute Risk Reduction (‘ARR’). ARR compares the impact of treatment by comparing the outcomes of the treated group and the untreated group. In other words, if 20 out of 100 untreated individuals had a negative outcome, and 10 out of 100 treated individuals had a negative outcome, the ARR would be 10% (20 - 10 = 10). According to a study published by the NIH, the ARR for the Pfizer Vaccine is a mere 0.7%, and the ARR for the Moderna Vaccine is only 1.1%...
(4) § 360bbb–3(c)(2)(B): The Known and Potential Risks of the Vaccine Outweigh their Known and Potential Benefits…
The typical vaccine development process takes between 10 and 15 years, and consists of the following sequential stages: research and discovery (2 to 10 years), pre-clinical animal studies (1 to 5 years), clinical human trials in four phases (typically 5 years). Phase 1 of the clinical human trials consists of healthy individuals and is focused on safety. Phase 2 consists of additional safety and dose-ranging in healthy volunteers, with the addition of a control group. Phase 3 evaluates efficacy, safety and immune response in a larger volunteer group, and requires two sequential randomized controlled trials. Phase 4 is a larger scale investigation into longer- term safety. Vaccine developers must follow this process in order to be able to generate the data the FDA needs in order to assess the safety and effectiveness of a vaccine candidate.
This 10-15 year testing process has been abandoned for purposes of the Vaccines. The first human-to-human transmission of the SARS-CoV-2 virus was not confirmed until January 20, 2020, and less than a year later both mRNA Vaccines had EUAs and for the first time in history this novel mRNA technology was being injected into millions of human beings…
All of the stages of testing have been compressed in time, abbreviated in substance, and are overlapping, which dramatically increases the risks of the Vaccines. Plaintiffs’ investigation indicates that Moderna and Pfizer designed their Vaccines in only two days. It appears that pharmaceutical companies did not independently verify the genome sequence that China released on January 11, 2020. It appears that the Vaccines were studied for only 56 days in macaques, and 28 days in mice, and then animal studies were halted. It appears that the pharmaceutical companies discarded their control groups receiving placebos, squandering the opportunity to learn about the rate of long-term complications, how long protection against the disease lasts and how well the Vaccines inhibit transmission. A number of studies were deemed unnecessary and not performed prior to administration in human subjects, including single dose toxicity, toxicokinetic, genotoxicity, carcinogenicity, prenatal and postnatal development, offspring, local tolerance, teratogenic and postnatal toxicity and fertility. The American public has not been properly informed of these dramatic departures from the standard testing process, and the risks they generate.
Plaintiff America’s Frontline Doctors’ ('AFLDS') medico-legal researchers have analyzed the accumulated COVID-19 Vaccine risk data, and report as follows: ...”
Further content:
- Migration of the SARS-CoV-2 “Spike Protein” in the Body (p. 11)
- Increased Risk of Death from Vaccines (p. 12)
- Reproductive Health (pp. 12-14)
- Vascular Disease (pp. 14-15)
- Autoimmune Disease (p. 15)
- Neurological Damage (pp. 15-17)
- Effect on the Young (pp. 17-18)
- Chronic Disease (p. 18)
- Antibody Dependent Enhancement (pp. 18-19)
- Vaccine-Driven Disease Enhancement in the Previously Infected (pp. 38-40)
- More Virulent Strains (p. 20)
- Blood Supply (pp. 20-21)
Link for all documentation related to the case:
https://www.courtlistener.com/docket/59929233/americas-frontline-doctors-etc-v-becerra/
Plaintiffs motion in US District Court. Works of the United States government are not entitled to domestic copyright protection under U.S. law and are therefore in the public domain.
