¨Abstract
This paper reviews safety and reactogenicity data for the BNT162 vaccine candidates, including BNT162b2 (Comirnaty, Pfizer/BioNTech COVID-19 vaccine) and bivalent variant-adapted BNT162b2 vaccines, from preclinical studies, clinical trials, post-marketing surveillance, and real-world studies, including an unprecedentedly large body of independent evidence...
Introduction
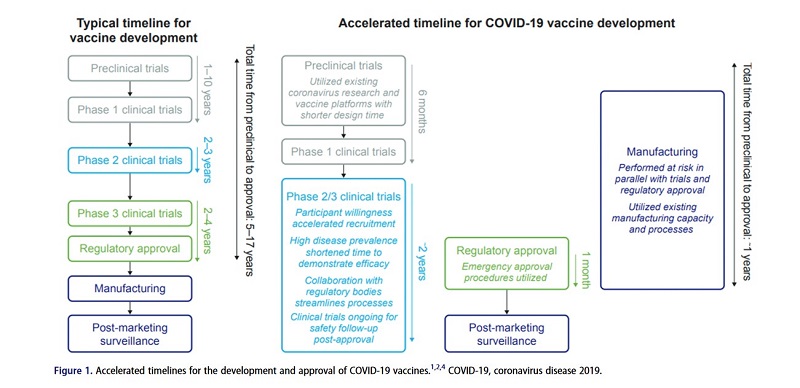
... Pre-existing manufacturing processes for messenger RNA (mRNA) vaccines enabled rapid production and scale-up. Subsequently, development and commercialization timelines were much shorter compared with other vaccines, such as influenza, while every step of the pathway required for regulatory approval was fulfilled...
Results ...
Phase II/III clinical trials of original BNT162b2
The pivotal Phase II/III clinical trial of BNT162b2 (NCT04368728) was a continuation of the Phase I trial in the United States, expanded to a global scope. This trial randomized 43,548 participants ≥16 years of age to receive two doses of 30 μg BNT162b2 or placebo, and included participants from 130 sites in the United States, Argentina, Brazil, South Africa, Germany, and Turkey. The overall safety population included 18,860 participants who received BNT162b2. In total, 58% were 16–55 years of age and 42% were >55 years of age...
Systemic events were more frequent after the second dose and in younger (16–55 years of age) versus older (≥55 years of age) participants. In total, ≤2% of participants vaccinated with BNT162b2 reported solicited systemic events graded as severe within 7 days of administration, with the exception of fatigue, which occurred in 3.8% of participants after dose 2...
Selected AESIs reported with BNT162b2 vaccination
... AESIs [adverse events of special interest] that have been reported following BNT162b2 vaccination include Bell’s palsy, and myocarditis and pericarditis."
¨Disclosure statement
ÖT is a management board member and employee at BioNTech SE (Mainz, Germany) and co-founder of the company. FvO, NC, FJM, CL, SP, and RR are employees at BioNTech SE. ÖT is an inventor on patents and patent applications related to RNA technology and COVID-19 vaccines. SP, RR, CL and ÖT have securities from BioNTech SE.
Funding
The work was supported by the BioNTech¨
This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. The terms on which this article has been published allow the posting of the Accepted Manuscript in a repository by the author(s) or with their consent.
