"Abstract
Introduction: ... This study aimed to identify the patterns associated with serious AE [adverse event] reports after mRNA COVID-19 vaccination in the World Health Organization (WHO)’s global scale database (VigiBase).
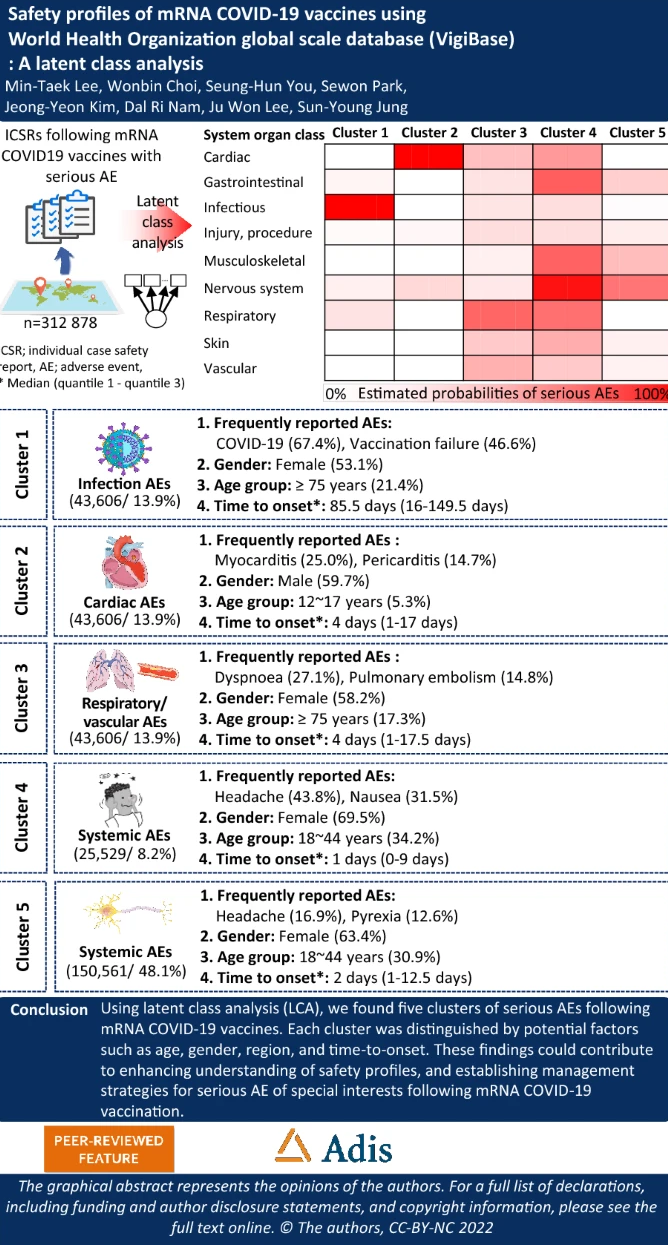
Methods: This study performed a latent class analysis (LCA) of reports of serious AEs following mRNA COVID-19 vaccination from VigiBase between December 28, 2020 , and February 28, 2022 (N = 312878)...
Results: Five clusters of AE reports were distinguished through LCA: infection AEs (cluster 1), cardiac AEs (cluster 2), respiratory / thrombotic AEs (cluster 3), systemic AEs (cluster 4), and nervous system AEs (cluster 5)...
Discussion
... We identified five distinguished safety profiles using LCA and described the potential factors of serious AEFI [adverse events following immunization] profiles, which included age, gender, region, and TTO [time to onset].
We suggest that cluster 1 (infection AEs) might be related to the ineffectiveness of mRNA vaccines. The most common PTs [Preferred Term] of cluster 1 were COVID-19 infection and vaccination failure...
We identified myocarditis and pericarditis following mRNA COVID-19 vaccination in cluster 2 (cardiac AEs). This association was not reported in the trial...
[C]luster 3 showed a high proportion of vascular disorders, including pulmonary embolism, deep vein thrombosis, and thrombosis, along with respiratory disorders...
In cluster 5, reports of headache and dizziness without cardiac and respiratory disorders made up a high proportion. We identified 3301 reports of facial paralysis (85.7% of total reports of facial paralysis) and 1494 reports of Guillain-Barré syndrome (82.6% of total reports of facial paralysis), which are rare but serious events."
© The Author(s) 2022
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
