"1 Introduction
... [S]erious or rare adverse reactions have been reported. BNT162b2 [Pfizer] and mRNA-1273 [Moderna] vaccines can cause serious allergic reactions, usually within 30 min of vaccination; therefore, contraindications and history of allergy should be determined in advance, and adequate amounts of epinephrine should be available. Serious adverse reactions associated with the Ad26.COV2.S [Johnson & Johnson] vaccine include serious skin adverse reactions, Bell’s palsy, and anxiety...
The serious adverse events following immunization (AEFI) reported concerning the COVID-19 vaccines also include thrombosis, thrombocytopenia, and several cases of unusual thrombotic events in combination with thrombocytopenia. The vast majority of reported immune thrombotic thrombocytopenia (VITT) cases have occurred following adenoviral vector-based vaccination with ChAdOx1nCoV-19 (Oxford/AstraZeneca) or Ad26.COV2.S...
The U.S. Food and Drug Administration (FDA)’s Vaccine Adverse Event Reporting System (VAERS) is the database for vaccines. The VAERS was used to obtain data, and statistical methods such as the reporting odds ratio (ROR) were used to process the collected data to discover the various adverse reaction signals of the aforementioned three COVID-19 vaccines, especially serious AEFI...
3.1 Descriptive analyses of serious AEFI related to BNT162b2, Ad26.COV2.S, and mRNA-1273
A total of 445,926, 167,457, and 535,126 cases of AEFI were reported after vaccination with BNT162b2, Ad26.COV2.S, and mRNA-1273, respectively. Among the serious AEFI associated with the COVID-19 vaccine, people who died after the vaccination accounted for 1,963 cases (4.40 per 1,000, 1,963/445,926) for BNT162b2, 345 cases (2.06 per 1,000, 345/167,457) for Ad26.COV2.S, and 2,077 cases (3.88 per 1,000, 2,077/535,126) for mRNA-1273...
Among the 1,963 people who died after receiving the BNT162b2 vaccine, the most common combined AEFI were dyspnea (accounting for 12.48% of the 1,963 people who died after the vaccination), cardiac arrest (8.51%), and lack of response to stimuli (7.74%)...
5 Conclusion
In conclusion, a disproportionality analysis was performed using the ROR method based on the VAERS data. Ad26.COV2.S vaccination was associated with a lower death frequency than BNT162b2 and mRNA-1273. Ad26.COV2.S vaccination was associated with a lower birth defect and emergency room visit frequency than BNT162b2... Patients with underlying medical conditions may be advised to use the aforementioned information for vaccination selection. These results are consistent with those of previous studies."
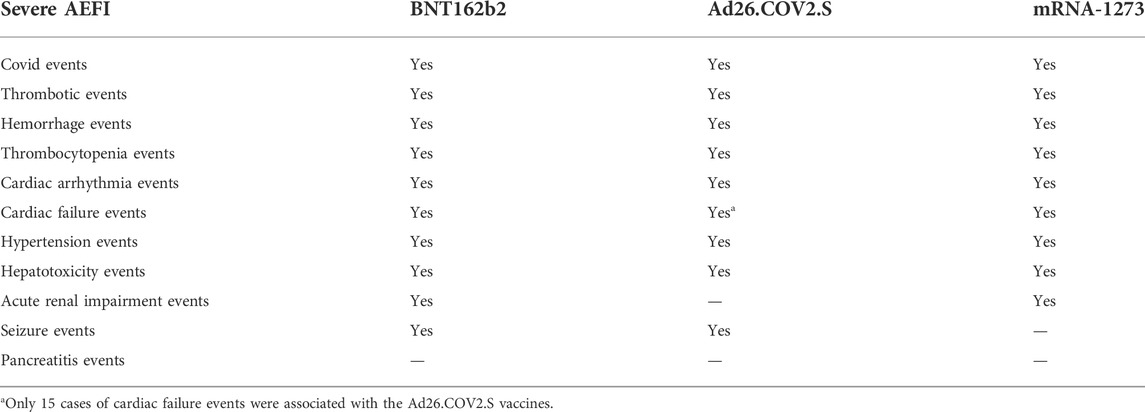
Table 1. Severe AEFI associated with the BNT162b2, Ad26.COV2.S, and mRNA-1273 vaccines
© 2022 Yan, Zhao, Li, Chow, Zhang, Qi, Wu, Zhong and Qiu.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
