"What were the main outcomes after 2-year of implementation of the French enhanced national PV [pharmocovigilance] system?
From December 21, 2020 to December 31, 2022, in France, more than 155 million injections of COVID-19 vaccines were performed... In total, 190,000 cases of adverse event following immunization reports (25% serious, 75% non-serious) were documented, analyzed and recorded in the French national pharmacovigilance database by the 30 Regional Pharmacovigilance Centres: 124,647 cases (26% serious, 74% non-serious) with Comirnaty®, 33,115 cases (20% serious, 80% non-serious) with Spikevax®, 31,391 cases (23% serious, 77% non-serious) with Vaxzevria®, 1723 cases (36% serious, 64% non-serious) with Jcovden® and 92 cases (25% serious, 75% non-serious) with Nuvaxovid®...
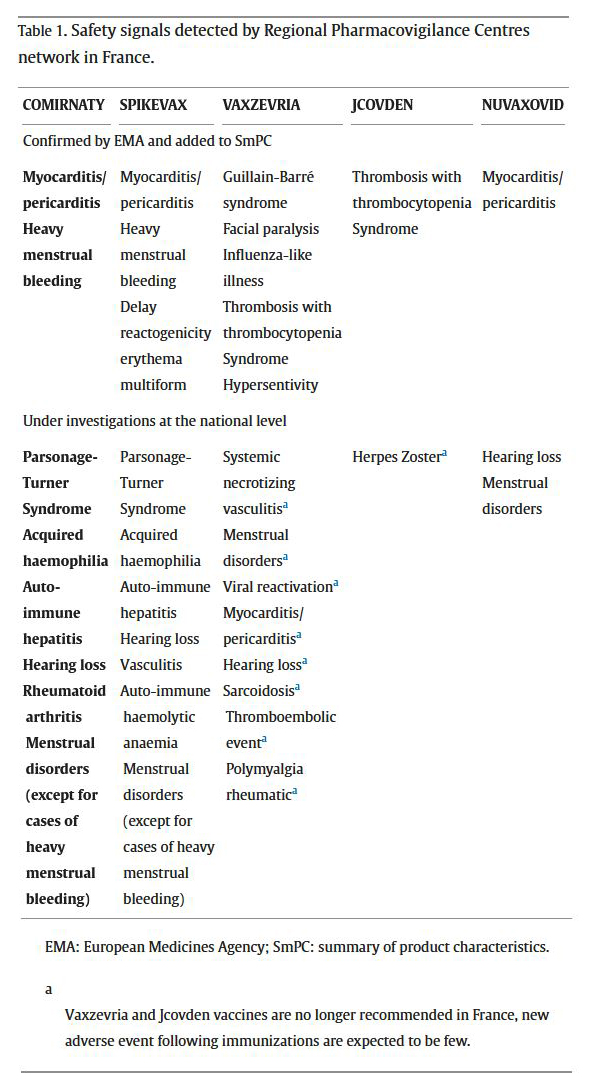
After expertise of the 190,000 cases of adverse event following immunization, the Regional Pharmacovigilance Centres identified and transmitted to the ANSM [French National Agency for Medicines and Health Products Safety] 1153 'remarkable reports' related to the vaccines. Eighty-two were retained by the experts and the monitoring committee as potential safety signals, and 53 were escalated to the PRAC [Pharmacovigilance Risk Assessment Committee] for confrontation with other EU pharmacovigilance reports and collective discussion with all PRAC members. From these, 13 have been confirmed by the EMA [European Medicines Agency] and added to the summary of product characteristics of these vaccines. Among them, some signals were specific to a type of vaccine (or ARNm) such as major flu-like syndrome, facial paralysis and Guillain-Barre syndrome or thrombosis associated with thrombocytopenia for adenovirus-based vaccines (for Vaxzevria® and Jcovden®), heavy menstrual bleeding for ARNm (Comirnaty® and Spikevax®), whereas others were reported for multiple type of vaccines, such as myocarditis/pericarditis for Comirnaty®, Spikevax® and Nuvaxovid®. Twenty-four safety signals are still under investigation such as hearing loss for mRNA vaccines...
Lessons Learnt
... Notably, signal detection (n = 53) at a national level, adjudication at the European level, communication to the public and to the scientific community, and translation into updated vaccine recommendation were highly coordinated. For instance, the safety signal for myocarditis/pericarditis with mRNA vaccines was detected in France at the end of April 2021. By early June 2021, a first recommendation on the ANSM website was published to warn the general public and healthcare professionals about this risk. In early November 2021, after an in-depth and detailed analysis of the cases of myocarditis/pericarditis reported with Spikevax®, the Regional Pharmacovigilance Centres further identified that this effect occurred more frequently in young adult (< 30 years) than with Comirnaty®. This new signal was confirmed by a pharmacoepidemiological study. A French national immunization technical advisory groups's recommendation was immediately published, specifying that only Comirnaty® should be used in patients under 30 years of age. France was also one of the main contributors to the European analysis of the heavy menstrual bleeding signal with mRNA vaccines, leading the PRAC to validate this signal in October 2022."
© 2024 The Author(s). Published by Elsevier Masson SAS on behalf of Societ ´ e´ franc¸aise de pharmacologie et de therapeutique.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
