October 22, 2020
Food and Drug Administration (FDA)
VRBPAC Meeting
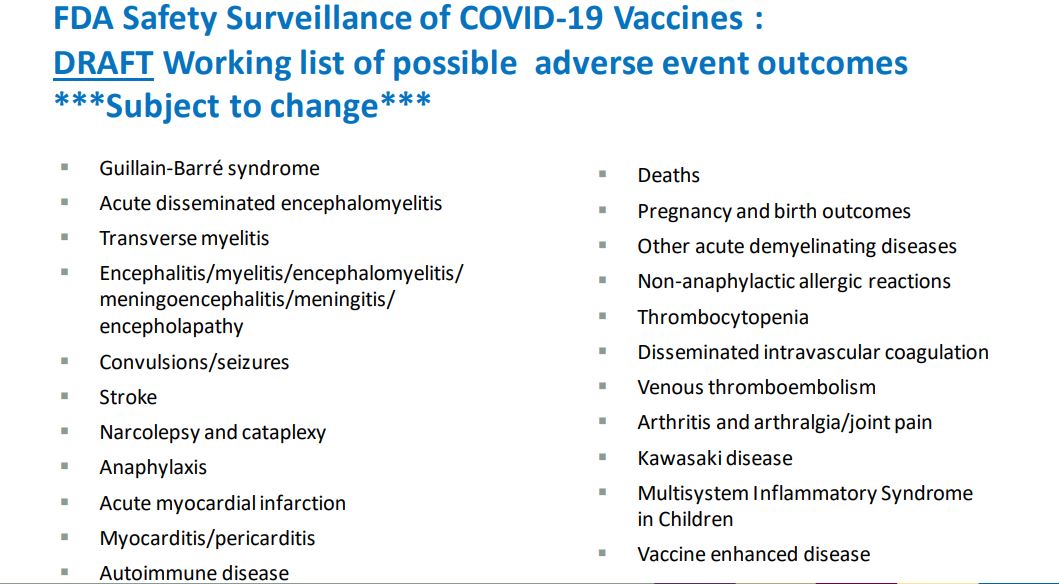
"FDA Safety Surveillance of COVID-19 Vaccines: DRAFT Working list of possible adverse event outcomes:
- Guillain-Barré syndrome
- Acute disseminated encephalomyelitis
- Transverse myelitis
- Encephalitis/myelitis/encephalomyelitis/meningoencephalitis/meningitis/encepholapathy
- Convulsions/seizures
- Stroke
- Narcolepsy and cataplexy
- Anaphylaxis
- Acute myocardial infarction
- Myocarditis/pericarditis
- Autoimmune disease
- Deaths
- Pregnancy and birth outcomes
- Other acute demyelinating diseases
- Non-anaphylactic allergic reactions
- Thrombocytopenia
- Disseminated intravascular coagulation
- Venous thromboembolism
- Arthritis and arthralgia/joint pain
- Kawasaki disease
- Multisystem Inflammatory Syndrome in Children
- Vaccine enhanced disease
Steve Anderson is the Director of the FDA's Office of Biostatistics and Epidemiology
Works of the United States government are not entitled to domestic copyright protection under U.S. law and are therefore in the public domain.
document
adverse events,autoimmunity,COVID-19,heart disorders,immunodeficiency and immunopathological disorders,neurological disorders,reproductive system issues,vaccines,vascular system issues
